Zolpidem
From Wikipedia, the free encyclopedia
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
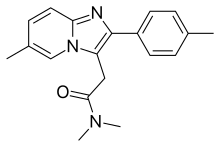
N,N-dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide
| |
| Clinical data | |
| Trade names | originally Ambien, many names worldwide[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693025 |
| Pregnancy category | |
| Dependence liability | High |
| Routes of administration | Oral (tablet), sublingual, oromucosal (spray), rectal |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70% (oral) |
| Protein binding | 92% |
| Metabolism | Liver through CYP3A4 |
| Biological half-life | 2–3 hours |
| Duration of action | 3 hours |
| Excretion | Kidney (56%) fecal (34%) |
| Identifiers | |
| CAS Number | 82626-48-0 |
| ATC code | N05CF02 (WHO) |
| PubChem | CID 5732 |
| IUPHAR/BPS | 4362 |
| DrugBank | DB00425 |
| ChemSpider | 5530 |
| UNII | 7K383OQI23 |
| KEGG | D08690 |
| ChEBI | CHEBI:10125 |
| ChEMBL | CHEMBL911 |
| Chemical data | |
| Formula | C19H21N3O |
| Molar mass | 307.395 g/mol |
| | |
Zolpidem (originally marketed as Ambien and available worldwide under many brand names)[1] is a sedative primarily used for the treatment of insomnia. It works quickly, usually within 15 minutes, and has a short half-life of two to three hours. Zolpidem has not adequately demonstrated effectiveness in maintaining sleep, unless delivered in a controlled-release (CR) form. However, it is effective in initiating sleep.[2] Its hypnotic effects are similar to those of the benzodiazepine class of drugs.
In 2013, the Food and Drug Administration required manufacturer decrease the recommended dose for women by half, after studies showed that the medicines can leave people drowsy in the morning and at risk for motor vehicle collisions. The FDA recommended that manufacturers extend the new dosage cuts to men as well, who process the drug at a faster rate; however, the reasons men and women metabolize the drugs at different rates are still unknown.[3] In May 2013, the FDA approved label changes specifying new dosage recommendations for zolpidem products because of concerns regarding next-morning impairment.[4] The underlying mechanism involve GABA.
It is a short-acting nonbenzodiazepine compound of the imidazopyridine class[5] that increases the activity of GABA, an inhibitory neurotransmitter, by binding to GABAAreceptors at the same location as benzodiazepines.[6] It is molecularly distinct from the classical benzodiazepine molecule and is classified as an imidazopyridine.Flumazenil, a benzodiazepine receptor antagonist, which is used for benzodiazepine overdose, can also reverse zolpidem's sedative/hypnotic and memory-impairing effects.[7][8]
The United States patent for zolpidem was held by the French pharmaceutical corporation Sanofi-Aventis.[9] On April 23, 2007, the U.S. Food and Drug Administration(FDA) approved 13 generic versions of zolpidem tartrate.[10] Zolpidem is available from several generic manufacturers in the UK, as a generic from Sandoz in South Africa and TEVA in Israel, as well as from other manufacturers such as Ratiopharm and Takeda GmbH (both Germany).
Medical uses[edit]
Sleep[edit]
Clinicians prescribe zolpidem for short-term (usually about two to six weeks) treatment of insomnia.[11] Zolpidem addresses sleep-initiation problems, but is not effective in maintaining sleep.[2] Also, a 2012 NIH study showed that zolpidem's effectiveness is nearly as much due to psychological effects as to the drug itself, so "increased attention should be directed at psychological intervention of insomnia."[12]
Other[edit]
Zolpidem has some muscle relaxant and anticonvulsant properties, but has not been approved for use in muscle relaxation or seizure prevention. This is because the dosage of drug needed to cause muscle relaxation is 10 times the sedating dose, while early studies indicated that the dosage needed for preventing seizures is 20 times the sedating dose.[13]
Adverse effects[edit]
The most common side effects for short term use include headache (reported by 7% of people in clinical trials) drowsiness (2%), dizziness (1%), and diarrhea (1%); the most common side effects of long term use included dry mouth (3%), allergy (4%), back pain (3%), flu-like symptoms (1%), chest pain (1%), heart palpitations (2%), drowsiness (8%), dizziness (5%), lethargy (3%), drugged feeling (3%), lightheadedness (2%), depression (1%), abnormal dreams (1%), amnesia (1%), sleep disorder (1%), diarrhea (3%), abdominal pain (2%), constipation (2%), sinusitis (4%), sore throat (3%), and rash (2%).[5]
Some users have reported unexplained sleepwalking[14] while using zolpidem, as well as sleep driving,[14] Night eating syndrome while asleep, and performing other daily tasks while sleeping. Research by Australia's National Prescribing Service found these events occur mostly after the first dose taken, or within a few days of starting therapy.[15] Rare reports of sexual parasomnia episodes related to zolpidem intake have also been reported.[16] Sleepwalkers can sometimes perform these tasks as normally as they might if they were awake.
Residual 'hangover' effects, such as sleepiness and impaired psychomotor and cognitive function, may persist into the day following nighttime administration. Such effects may impair the ability of users to drive safely and increase risks of falls and hip fractures.[14][17]
In February 2008, the Australian Therapeutic Goods Administration attached a boxed warning to zolpidem, stating that "Zolpidem may be associated with potentially dangerous complex sleep-related behaviors that may include sleep walking, sleep driving, and other bizarre behaviours. Zolpidem is not to be taken with alcoholic beverages. Caution is needed with other CNS-depressant drugs. Limit use to four weeks maximum under close medical supervision."[18]
Tolerance, dependence, and withdrawal[edit]
A review medical publication found long-term use of zolpidem is associated with drug tolerance, substance dependence, rebound insomnia, and CNS-related adverse effects. It was recommended that zolpidem be used for short periods of time using the lowest effective dose. Zolpidem 10 mg is effective in treating insomnia when used intermittently no fewer than three and no more than five pills per week for a period of 12 weeks.[19] The 15-mg zolpidem dosage provided no clinical advantage over the 10-mg zolpidem dosage.[20]
Nonpharmacological treatment options (e.g. cognitive behavioral therapy for insomnia), however, were found to have sustained improvements in sleep quality.[21] Animal studies of the tolerance-inducing properties have shown that in rodents, zolpidem has less tolerance-producing potential than benzodiazepines, but in primates the tolerance-producing potential of zolpidem was the same as that of benzodiazepines.[22] Tolerance to the effects of zolpidem can develop in some people in just a few weeks. Abrupt withdrawal may cause delirium, seizures, or other severe effects, especially if used for prolonged periods and at high dosages.[23][24][25]
When drug tolerance and physical dependence to zolpidem has developed, treatment usually entails a gradual dose reduction over a period of months to minimise withdrawal symptoms, which can resemble those seen during benzodiazepine withdrawal. Failing that, an alternative method may be necessary for some patients, such as a switch to abenzodiazepine equivalent dose of a longer-acting benzodiazepine drug, such as diazepam or chlordiazepoxide, followed by a gradual reduction in dosage of the long-actingbenzodiazepine. Sometimes for difficult-to-treat patients, an inpatient flumazenil rapid detoxification program can be used to detoxify from a zolpidem drug dependence oraddiction.[26]
Alcohol has cross tolerance with GABAA receptor positive modulators such as the benzodiazepines and the nonbenzodiazepine drugs. For this reason, alcoholics or recovering alcoholics may be at increased risk of physical dependency on zolpidem. Also, alcoholics and drug abusers may be at increased risk of abusing and or becoming psychologically dependent on zolpidem. It should be avoided in those with a history of alcoholism, drug misuse, physical dependency, or psychological dependency on sedative-hypnotic drugs. Zolpidem has rarely been associated with drug-seeking behavior,[citation needed] the risk of which is amplified in patients with a history of drug or alcohol abuse.
Overdose[edit]
An overdose of zolpidem may cause excessive sedation, pin-point pupils, or depressed respiratory function, which may progress to coma, and possibly death. Combined with alcohol, opiates, or other CNS depressants, it may be even more likely to lead to fatal overdoses. Zolpidem overdosage can be treated with the benzodiazepine receptor antagonist flumazenil, which displaces zolpidem from its binding site on the benzodiazepine receptor to rapidly reverse the effects of the zolpidem.[27]
Detection in body fluids[edit]
Zolpidem may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients, provide evidence in an impaired driving arrest, or to assist in a medicolegal death investigation. Blood or plasma zolpidem concentrations are usually in a range of 30–300 μg/l in persons receiving the drug therapeutically, 100–700 μg/l in those arrested for impaired driving, and 1000–7000 μg/l in victims of acute overdosage. Analytical techniques, in general, involve gas or liquid chromatography.[28][29][30]
Special precautions[edit]
Driving[edit]
Use of zolpidem may impair driving skills with a resultant increased risk of road traffic accidents. This adverse effect is not unique to zolpidem but also occurs with other hypnotic drugs. Caution should be exercised by motor vehicle drivers.[31] Studies showed that eight hours after a bedtime dose of 10 mg, 15% of women and 3% of men would have blood levels that produce impaired driving skills; for an extended-release dose of 12.5 mg, the risk increased to 33% and 25%, respectively. As a consequence, the FDA recommended the dose for women be reduced and that prescribers should consider lower doses for men.[32][33]
Elderly[edit]
The elderly are more sensitive to the effects of hypnotics including zolpidem. Zolpidem causes an increased risk of falls and may induce adverse cognitive effects.[34]
An extensive review of the medical literature regarding the management of insomnia and the elderly found that there is considerable evidence of the effectiveness and durability of nondrug treatments for insomnia in adults of all ages, and these interventions are underused. Compared with the benzodiazepines, the nonbenzodiazepine (including zolpidem) sedative-hypnotics appeared to offer few, if any, significant clinical advantages in efficacy or tolerability in elderly persons. Newer agents with novel mechanisms of action and improved safety profiles, such as the melatonin receptor agonists, were found to hold promise for the management of chronic insomnia in elderly people.
Long-term use of sedative-hypnotics for insomnia lacks an evidence base and has traditionally been discouraged for reasons that include concerns about such potential adverse drug effects as cognitive impairment (anterograde amnesia), daytime sedation, motor incoordination, and increased risk of motor vehicle accidents and falls. In addition, the effectiveness and safety of long-term use of these agents remain to be determined. More research is needed to evaluate the long-term effects of treatment and the most appropriate management strategy for elderly persons with chronic insomnia.[35]
Gastroesophageal reflux disease[edit]
Patients suffering from gastroesophageal reflux disease (GERD) had reflux events measured to be significantly longer when taking zolpidem than on placebo. The same trend was found for reflux events in patients without GERD. This is assumed to be due to suppression of arousal during the reflux event, which would normally result in a swallowing reflex to clear gastric acid from the esophagus. Patients with GERD experience significantly higher esophageal exposure to gastric acid, which increases the likelihood of their developing esophageal cancer.[36]
Pregnancy[edit]
Zolpidem has been assigned to pregnancy category C by the FDA. Animal studies have revealed evidence of incomplete ossification and increased postimplantation fetal loss at doses greater than seven times the maximum recommended human dose or higher; however, teratogenicity was not observed at any dose level. There are no controlled data in human pregnancy. In one case report, zolpidem was found in cord blood at delivery. Zolpidem is recommended for use during pregnancy only when benefits outweigh risks. [37]
Mechanism of action[edit]
Zaleplon and zolpidem both are agonists at the GABA A α 1 subunit. Due to its selective binding, zolpidem has very weak anxiolytic, myorelaxant, and anticonvulsant properties but very strong hypnotic properties.[38] Zolpidem binds with high affinity and acts as a full agonist at the α1-containing GABAA receptors, about 10-fold lower affinity for those containing the α2- and α3- GABAA receptor subunits, and with no appreciable affinity for α5 subunit-containing receptors.[39][40] ω1 type GABAA receptors are the α1-containing GABAA receptors and ω2 GABAA receptors are the α2-, α3-, α4-, α5-, and α6-containing GABAA receptors. ω1 GABAA receptors are found primarily in the brain, whereas ω2 receptors are found primarily in the spine. Thus, zolpidem has a preferential binding for the GABAA-benzodiazepine receptor complex in the brain but a low affinity for the GABAA-benzodiazepine receptor complex in the spine.[41]
Like the vast majority of benzodiazepine-like molecules, zolpidem has no affinity for α4 and α6 subunit-containing receptors.[42] Zolpidem positively modulates GABAA receptors, it is presumed by increasing the GABAA receptor complex's apparent affinity for GABA without affecting desensitization or peak current.[43] Like zaleplon (Sonata), zolpidem may increase slow wave sleep but cause no effect on stage 2 sleep.[44]
A meta-analysis of the randomised, controlled, clinical trials that compared benzodiazepines against nonbenzodiazepines such as zolpidem has shown few consistent differences between zolpidem and benzodiazepines in terms of sleep onset latency, total sleep duration, number of awakenings, quality of sleep, adverse events, tolerance, rebound insomnia, and daytime alertness.[45]
Chemistry[edit]
Three syntheses of zolpidem are common. 4-methylacetophenone is used as a common precursor. This is brominated and reacted with 2-amino-5-methylpyridine to give the imidazopyridine. From here the reactions use a variety of reagents to complete the synthesis, either involving thionyl chloride or sodium cyanide. These reagents are challenging to handle and require thorough safety assessments.[46][47][48]Though such safety procedures are common in industry, they make clandestine manufacture difficult.
A number of major side-products of the sodium cyanide reaction have been characterised and include dimers and mannich products.[49]
Drug–drug interactions[edit]
Notable drug–drug interactions with the pharmacokinetics of zolpidem include chlorpromazine, fluconazole, imipramine, itraconazole, ketoconazole, rifampicin, and ritonavir. Interactions with carbamazepine andphenytoin can be expected based on their metabolic pathways, but have not yet been studied. There does not appear to be any interaction between zolpidem and cimetidine or ranitidine.[50][51] However, it was noted in the same study that cimetidine did appear to prolong the hypnotic effects of Zolpidem beyond its typical 3 hour duration, which is indicative of some sort of metabolic interaction.[50]
Usage[edit]
Zolpidem is one of the most common GABA-potentiating sleeping medications prescribed in the Netherlands, with a total of 582,660 prescriptions dispensed in 2008.[52]
The United States Air Force uses zolpidem as one of the hypnotics approved as a "no-go pill" (with a 6-hour restriction on subsequent flight operation) to help aviators and special duty personnel sleep in support of mission readiness. (The other hypnotics used are temazepam and zaleplon.) "Ground tests" are required prior to authorization issued to use the medication in an operational situation.[53]
Society and culture[edit]
Abuse[edit]
Zolpidem has a potential for either medical misuse when the drug is continued long term without or against medical advice or recreational use when the drug is taken to achieve a "high".[54] The transition from medical use of zolpidem to high-dose addiction or drug dependence can occur when used without a doctor's recommendation to continue using it, when physiological drug tolerance leads to higher doses than the usual 5 mg or 10 mg, when consumed through inhalation or injection, or when taken for purposes other than as a sleep aid. Misuse is more prevalent in those having been dependent on other drugs in the past, but tolerance and drug dependence can still sometimes occur in those without a history of drug dependence. Chronic users of high doses are more likely to develop physical dependence on the drug, which may cause severe withdrawal symptoms, including seizures, if abrupt withdrawal from zolpidem occurs.[55]
One case history reported a woman detoxifying from a high dose of zolpidem experiencing a generalized seizure, with clinical withdrawal and dependence effects reported to be similar to the benzodiazepine withdrawal syndrome.[56]
Other drugs, including the benzodiazepines and zopiclone, are also found in high numbers of suspected drugged drivers. Many drivers have blood levels far exceeding the therapeutic dose range suggesting a high degree of excessive-use potential for benzodiazepines, zolpidem and zopiclone.[57] U.S. Congressman Patrick J. Kennedy says that he was using Zolpidem (Ambien) and Phenergan when caught driving erratically at 3AM.[58] "I simply do not remember getting out of bed, being pulled over by the police, or being cited for three driving infractions," Kennedy said.
Nonmedical use of zolpidem is increasingly common in the U.S., Canada, and the UK. Recreational users report that resisting the drug's hypnotic effects can in some cases elicit vivid visuals and a body high.[59]Some users have reported decreased anxiety, mild euphoria, perceptual changes, visual distortions, and hallucinations.[60]
Zolpidem (Stilnox) was used by Australian Olympic swimmers at the London Olympics in 2012, leading to controversy.[61]
Regulation[edit]
Zolpidem, along with the other benzodiazepine-like Z-drugs, is a Schedule IV controlled substance in the U.S., according to the Controlled Substances Act, given its potential for abuse and dependence.
Date rape drug[edit]
Zolpidem has become one of many date rape drug.[62][63] Unlike Rohypnol ("roofies"), which was banned in 1996, zolpidem is available legally by prescription, and unlike gamma-hydroxybutyrate, which is used to treat a rare form of narcolepsy, zolpidem was prescribed 43.8 million times in the U.S. in 2012.[62] It dissolves readily in liquids such as wine,[62] and can typically be detected in bodily fluids for only 36 hours, though it may be possible to detect it by hair testing much later;[62] this is due to the short elimination half-life of 2.5–3 hours.[64] This application of the drug was highlighted during proceedings against Darren Sharper, who was accused of using the tablets he was prescribed to facilitate a series of rapes.[62][63]
Sleepwalking[edit]
Zolpidem received widespread media coverage in Australia after the death of a student who fell 20 m from the Sydney Harbour Bridge while under the influence of zolpidem.[65]
Research[edit]
Zolpidem may provide short-lasting but effective improvement in symptoms of aphasia present in some survivors of stroke. The mechanism for improvement in these cases remains unexplained and is the focus of research by several groups, to explain how a drug that acts as a hypnotic-sedative in people with normal brain function, can increase speech ability in people recovering from severe brain injury. As of 2011 use of zolpidem for this application remains experimental,and is not officially approved by any pharmaceutical manufacturers of zolpidem or medical regulatory agencies worldwide.[66][67][68][69][70]
Zolpidem has been studied to determine whether it causes improved responsiveness or regional cerebral perfusion in patients with persistent vegetative states.[71][72][73][74]
The data suggest that the treatment shows a good response rate amongst patients whose brain damage was caused by stroke and also that the correlation between severity of the damage and likelihood of response to the drug is negative, being approximately 25% amongst lesser brain injury patients, whilst severely brain-damaged patients, such as those that are also in vegetative states, show a response of approximately 5% to 10%. However this trend in response does not appear to have any correlation with the effectiveness of the drug across different levels of injury severity.[75][76][77][78][79]
References[edit]
more information
Questions and Answers: Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist)
The U.S. Food and Drug Administration (FDA) is notifying the public of new information about zolpidem, a widely prescribed insomnia drug. FDA recommends that the bedtime dose be lowered because new data show that blood levels in some patients may be high enough the morning after use to impair activities that require alertness, including driving. Today’s announcement focuses on zolpidem products approved for bedtime use, which are marketed as generics and under the brand names Ambien, Ambien CR, Edluar, and Zolpimist.
FDA is also reminding the public that all drugs taken for insomnia can impair driving and activities that require alertness the morning after use. Drowsiness is already listed as a common side effect in the drug labels of all insomnia drugs, along with warnings that patients may still feel drowsy the day after taking these products. Patients who take insomnia drugs can experience impairment of mental alertness the morning after use, even if they feel fully awake.
FDA urges health care professionals to caution all patients (men and women) who use these zolpidem products about the risks of next-morning impairment for activities that require complete mental alertness, including driving. For zolpidem products, data show the risk for next-morning impairment is highest for patients taking the extended-release forms of these drugs (Ambien CR and generics). Women appear to be more susceptible to this risk because they eliminate zolpidem from their bodies more slowly than men.
Because use of lower doses of zolpidem will result in lower blood levels in the morning, FDA is requiring the manufacturers of Ambien, Ambien CR, Edluar, and Zolpimist to lower the recommended dose. FDA has informed the manufacturers that the recommended dose of zolpidem for women should be lowered from 10 mg to 5 mg for immediate-release products (Ambien, Edluar, and Zolpimist) and from 12.5 mg to 6.25 mg for extended-release products (Ambien CR). FDA also informed the manufacturers that, for men, the labeling should recommend that health care professionals consider prescribing the lower doses―5 mg for immediate-release products and 6.25 mg for extended-release products (see Zolpidem Dosing Recommendations for Adults).
The following questions and answers provide an overview of this safety issue.
Q1. What is zolpidem?
Q2. Why is FDA requiring the manufacturers of certain zolpidem-containing products to revise the labeling to lower the recommended dose of zolpidem for women and to recommend consideration of the lower dose in men?
Q3. What should patients currently taking the 10 mg or 12.5 mg dose of zolpidem-containing insomnia medicines do now?
Q4. Will a lower dose of zolpidem be effective in treating insomnia?
Q5. Is FDA requiring the manufacturer of Intermezzo (zolpidem tartrate) sublingual tablets to also change the dosing recommendations?
Q6. Do any other factors, such as a patient’s age, weight, or ethnicity, have an effect on zolpidem levels?
Q7. Why is FDA informing the public about this safety risk now, after zolpidem has been on the market for nearly 20 years?
Q8. Is next-morning impairment the same as complex sleep-related behaviors?
Q9. Is FDA requiring the manufacturers of other insomnia medicines to revise their dosing recommendations?
Q10. Do other insomnia medicines have the same gender effect as zolpidem?
Q11. Do over-the-counter (OTC) insomnia medicines that are available without a prescription have a risk of next-morning impairment?
Q12. What can patients do to decrease their risk of next-morning impairment with insomnia medicines?
Q13. How many reports of zolpidem and impaired driving has FDA received? Were these reports used as evidence to support the proposed new dosing recommendations for certain zolpidem-containing products?
Q2. Why is FDA requiring the manufacturers of certain zolpidem-containing products to revise the labeling to lower the recommended dose of zolpidem for women and to recommend consideration of the lower dose in men?
Q3. What should patients currently taking the 10 mg or 12.5 mg dose of zolpidem-containing insomnia medicines do now?
Q4. Will a lower dose of zolpidem be effective in treating insomnia?
Q5. Is FDA requiring the manufacturer of Intermezzo (zolpidem tartrate) sublingual tablets to also change the dosing recommendations?
Q6. Do any other factors, such as a patient’s age, weight, or ethnicity, have an effect on zolpidem levels?
Q7. Why is FDA informing the public about this safety risk now, after zolpidem has been on the market for nearly 20 years?
Q8. Is next-morning impairment the same as complex sleep-related behaviors?
Q9. Is FDA requiring the manufacturers of other insomnia medicines to revise their dosing recommendations?
Q10. Do other insomnia medicines have the same gender effect as zolpidem?
Q11. Do over-the-counter (OTC) insomnia medicines that are available without a prescription have a risk of next-morning impairment?
Q12. What can patients do to decrease their risk of next-morning impairment with insomnia medicines?
Q13. How many reports of zolpidem and impaired driving has FDA received? Were these reports used as evidence to support the proposed new dosing recommendations for certain zolpidem-containing products?
A. Zolpidem is a sedative-hypnotic (sleep) medicine that is used in adults for the treatment of insomnia. Zolpidem is available as an oral tablet (Ambien and generics), an extended-release tablet (Ambien CR and generics), a sublingual (under-the-tongue) tablet (Edluar), and an oral spray (Zolpimist).
Zolpidem is also available under the brand name Intermezzo, a lower dose sublingual tablet that is approved for use as needed for the treatment of insomnia when a middle-of-the-night awakening is followed by difficulty returning to sleep.
Q2. Why is FDA requiring the manufacturers of certain zolpidem-containing products to revise the labeling to lower the recommended dose of zolpidem for women and to recommend consideration of the lower dose in men?
A. FDA is requiring the manufacturers of certain zolpidem-containing products to revise the labeling to lower the recommended dose of zolpidem-containing medicines for women and to recommend that health care professionals consider prescribing the lower dose for men because next-morning blood levels of zolpidem may be high enough to impair activities that require alertness, including driving. Patients with high levels of zolpidem can be impaired even if they feel fully awake. Zolpidem is eliminated from the body more slowly in women, so the drug can stay in their systems longer than it does in men.
Q3. What should patients currently taking the 10 mg or 12.5 mg dose of zolpidem-containing insomnia medicines do now?
A. If you are currently taking the 10 mg or 12.5 mg dose of zolpidem-containing insomnia medicine, continue taking your prescribed dose as directed until you have contacted your health care professional to ask for instructions on how to safely continue to take your medicine. Each patient and situation is unique, and the appropriate dose should be discussed with your health care professional.
A. FDA has informed the manufacturers that the recommended dose of zolpidem for women should be lowered from 10 mg to 5 mg for immediate-release products (Ambien, Edluar, and Zolpimist) and from 12.5 mg to 6.25 mg for extended-release products (Ambien CR). For men, FDA has informed the manufacturers that the labeling should recommend that health care professionals consider prescribing these lower doses. These lower doses of zolpidem (5 mg for immediate-release products and 6.25 mg for extended-release products) will be effective in most women and many men.
Q5. Is FDA requiring the manufacturer of Intermezzo (zolpidem tartrate) sublingual tablets to also change the dosing recommendations?
A. No. When Intermezzo was FDA-approved in November 2011, the label already recommended a lower dosage in women compared to men. The recommended and maximum dose of Intermezzo is 1.75 mg for women and 3.5 mg for men, taken only once per night as needed if a middle-of-the-night awakening is followed by difficulty returning to sleep.
Q6. Do any other factors, such as a patient’s age, weight or ethnicity, have an effect on zolpidem levels?
A. Based on data from pharmacokinetic trials, no relationship was evident between the zolpidem blood level and patients’ body weight or ethnicity. In elderly patients, zolpidem blood levels can be higher, and the lower doses are already recommended. In contrast to younger patients, zolpidem blood levels in elderly patients are not affected by gender.
Q7. Why is FDA informing the public about this safety risk now, after zolpidem has been on the market for nearly 20 years?
A. Since the approval of zolpidem, FDA has been continually monitoring the drug’s safety profile. As more data became available, FDA continued to assess the benefits and risks of zolpidem treatment. Over the years, FDA has received reports of possible driving impairment and motor vehicle accidents associated with zolpidem; however, in most cases it was difficult to determine if the driving impairment was related to zolpidem or to specific zolpidem blood levels because information about time of dosing and time of the impairment was often not reported. Recently, data from clinical trials and driving simulation studies have become available that allowed FDA to better characterize the risk of driving impairment caused by specific blood levels of zolpidem and to recognize the increased risk of driving-impairing blood levels of zolpidem in women. This led FDA to require the manufacturers of certain zolpidem-containing products to revise the dosing recommendations.
A. No, they are different. Next-morning impairment occurs when patients are awake the next morning, but levels of the insomnia medicine in their blood remain high enough to impair activities that require alertness, including driving.
Complex sleep-related behaviors occur when patients get out of bed while not fully awake, and sleep walk or do an activity such as drive a car, prepare and eat food, make phone calls, or have sex.
Both problems are made worse by high levels of zolpidem. The changes that FDA is requiring to the dosing recommendations in the drug labeling are expected to decrease the risk of both next-morning impairment and complex sleep-related behaviors.
Q9. Is FDA requiring the manufacturers of other insomnia medicines to revise their dosing recommendations?
A. No. At this time, FDA is only requiring the manufacturers of certain zolpidem-containing products to revise their dosing recommendations.
FDA is continuing to evaluate ways to lower the risk of next-morning impairment with other insomnia medicines.
Q10. Do other insomnia medicines have the same gender effect as zolpidem?
A. FDA is currently evaluating other insomnia medicines to determine if they affect men and women differently.
A. FDA is currently evaluating other insomnia medicines to determine if they affect men and women differently.
Q11. Do over-the-counter (OTC) insomnia medicines that are available without a prescription have a risk of next-morning impairment?
A. Yes. OTC insomnia medicines also have a risk for next-morning impairment. FDA is not recommending that patients who are currently taking prescription insomnia medicines switch to OTC insomnia medicines.
Patients who drive or perform activities that require full alertness the next morning should discuss with their health care professional if the insomnia medicine they are using is right for them.
Q12. What can patients do to decrease their risk of next-morning impairment with insomnia medicines?
A. Patients can decrease their risk of next-morning impairment by taking the lowest dose of their insomnia medicine that treats their symptoms. It is important for patients to take their insomnia medicine exactly as prescribed. Taking a higher dose than prescribed or using more than one insomnia medicine is dangerous if patients drive or perform activities that require full alertness the next morning, even if the drugs are taken at the beginning of the night. In addition, patients should not take insomnia medicine intended for bedtime use if less than a full night’s sleep (7-8 hours) remains. Likewise, patients should not take Intermezzo, a zolpidem product that is approved for use in the middle of the night, if less than 4 hours of sleep remain.
Q13. How many reports of zolpidem and impaired driving has FDA received? Were these reports used as evidence to support the proposed new dosing recommendations for certain zolpidem-containing products?
A. FDA has received about 700 reports of zolpidem and “impaired driving ability and/or road traffic accident.” Following a zolpidem label change in 2007, which added information to the Warnings and Precautions section of the label about complex sleep-related behaviors, including sleep-driving (patients getting out of bed while not fully awake and driving), there was a great deal of media attention. Since such publicity tends to “stimulate” reporting, this led to the considerable number of reports of zolpidem and impaired driving that were submitted to FDA’s Adverse Event Reporting System (AERS) database.
However, while AERS reports generally can be helpful in evaluating safety concerns, these AERS reports for zolpidem lacked the information necessary to understand whether high morning blood levels of zolpidem were the cause of the reported impaired driving. Specifically, these reports often did not include the dose or time zolpidem was taken, the time of the accident, whether alcohol or other drugs were also taken, and whether and when blood levels of the drug were measured. It wasn’t until FDA received the new data on next-day blood levels and driving simulation studies that the apparent frequency of next-morning mental impairment was better identified.
Related Information
- FDA Drug Safety Communication: Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist)
- Some Sleep Drugs Can Impair Driving
- MedWatch Safety Alert: Zolpidem Containing Products-- FDA Requires Lower Recommended Doses



মন্তব্যসমূহ
একটি মন্তব্য পোস্ট করুন